no. of electrons in carbon|How to find the Number of Protons, Electrons, Neutrons for : Baguio In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Carbon (C). From the Periodic . 22 Years Old Pinay Walker Unli EUT 1K PHP Lang! Wag Lang sa Loob! 15.16K. 81%. Tropa Kong Chx Depressed Daw Kaya Niyaya Ko ng SEX. 2.40K. 100%. Syado Nag-Init, Sa Daan Palang Nilaplap si Marikit! 2.79K. 100%. Broken si Beshy, Cinomfort Sabay Kantot Ko. Hihi. 77.07K. 100%.
PH0 · Protons Neutrons & Electrons of All Elements (List
PH1 · How to find the Number of Protons, Electrons, Neutrons for
PH2 · How many Electrons does Carbon Have?
PH3 · Electron Configuration for Carbon (C)
PH4 · Carbon (C)
PH5 · Carbon
PH6 · 2.6: Protons, Neutrons, and Electrons in Atoms
PH7 · 2.2: Electron Configurations
PH8 · 1.8: Subatomic Particles
6,846 Las Vegas jobs available in Las Vegas, NV on Indeed.com. Apply to Receptionist, Crematory Operator, Claims Representative and more!
no. of electrons in carbon*******Mar 23, 2023 The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its .
The allotropes of carbon include graphite, one of the softest known substances, and diamond, the hardest naturally occurring substance. It bonds readily with other small atoms, including other carbon atoms, and is capable of forming multiple stable covalent bonds with suitable multivalent atoms. Carbon is a component element in the large majority of all chemical compounds, with abou.
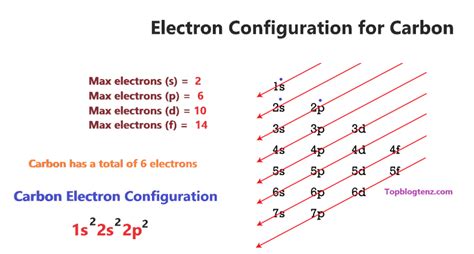
How to find the Number of Protons, Electrons, Neutrons for In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Carbon (C). From the Periodic .The element carbon (\(C\)) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. In a typical sample of carbon-containing material, 98.89% of the carbon atoms also contain 6 .Carbon occurs in all organic life and is the basis of organic chemistry. Carbon has the interesting chemical property of being able to bond with itself, and a wide variety of other elements. .no. of electrons in carbon How to find the Number of Protons, Electrons, Neutrons for Determine the number of protons and electrons in an atom. Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. Define . How many Electrons does Carbon Have? A neutral carbon atom has 6 electrons. In this article, we will study in detail the number of electrons carbon has. Table of Contents. 1. .By Hund’s rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state .Carbon is the sixth element with a total of 6 electrons. In writing the electron configuration for carbon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the .
Calculate the number of valence electrons in NO 2 . Because the double bonds are close enough to interact electronically with one another, the \(\pi\) electrons are shared over all the carbon atoms, as illustrated for 1,3 .
A Carbon-12 neutral atom has six protons, six neutrons, and six electrons. Carbon – 14 is a neutral element with six protons, eight neutrons, and six electrons. Some isotopes are stable, but others can emit or kick out subatomic particles in order to achieve a more stable, lower-energy configuration. These isotopes are referred to as . For example, a carbon atom weighs less than 2 \(\times\) 10 −23 g, and an electron has a charge of less than 2 \(\times\) 10 −19 C (coulomb). . Neutrons are relatively heavy particles with no charge and a mass of 1.0087 amu. Electrons are light particles with a charge of 1− and a mass of 0.00055 amu. The number of protons in the nucleus . To write the electron configuration for carbon, the first two electrons enter the 1s orbital, the next two electrons enter the 2s orbital, and the remaining two electrons enter the 2p subshell. Therefore, the electron configuration of carbon will be 1s 2 2s 2 2p 2. CO2 Molecular Geometry. The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other.The 3+ charge indicates that the element has lost 3 electrons. So, if the cation has 10 electrons, the neutral atom must have had 13 electrons because losing 3 of those results in a charge of 3+. And from here, we know that the element has 13 protons because the number of protons is equal to the number of electrons in a neutral atom.No. of atoms present in 1 mole of Carbon = N A = 6.023 × 10 23 As 1 mole of carbon contains 6 electrons, 6 protons and 6 neutrons. ∴ No. of electrons in 6.023 × 10 23 atoms of carbon = 6 × 6.023 × 10 23no. of electrons in carbon Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] . There are no electrons left to place on the central atom. 6. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form .In case of carbon dioxide, C has atomic number 6 so it has 6 electrons and oxygen has atomic no 8, i.e., 16 electrons. So total electrons present are 16+6=22 electrons.
CO 2 is the carbon dioxide's chemical formula. The sum of the electrons in carbon and oxygen is used to compute the total number of electrons in carbon dioxide. One carbon atom has 6 electrons, the number of electrons in two oxygen atoms is, 2 × 8 = 16 As a result, there are 6 + 16 = 22 total electrons in Carbon dioxide. To this end, we adopt a standard notation for each atom which displays the number of valence electrons in the unbonded atom explicitly. In this notation, carbon and hydrogen look like Figure 6.1, representing the single valence electron in hydrogen and the four valence electrons in carbon. Figure 6.1: Electron accounting notation for hydrogen .Calculate the number of electrons in 100 g r a m s of C O 2 Can a body have a charge of (a) 0.32 × 10 − 18 C (b) 0.64 × 10 − 20 C (c) 4.8 × 10 − 21 C ? View Solution
Carbon (atomic number 6) has six electrons. Four of them fill the 1s and 2s orbitals. . Electron configurations and orbital diagrams can be determined by applying the Pauli exclusion principle (no two electrons can . The element carbon (C) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. In a typical sample of carbon-containing material, 98.89% of the carbon atoms also contain 6 neutrons, so each has a mass number of 12. For example, the electron configuration of carbon atom is written as 1s 2,2s 2,2p 2 having 2 electrons in the K shell and 4 in the next L shell. Neon, on the other hand, has 10 electrons distributed as 2 each in 2s, and 3 p orbitals, with configuration 1s 2,2s 2,2p 6. Electron Spin. The electrons in an orbital have opposite spins.The General Rule. Atoms tend to have all its valence orbitals occupied by paired electrons. For transition metals, the valence orbitals consist of ns, 3 np and 5 (n-1)d orbitals, leading to its tendency of being surrounded by 18 electrons.

based on the electronic configaration of carbon, it has two unpaired electrons present. Hence, option B is correct. For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
Read the latest manager specials tips and news from our experts here at Betfair™! Next Manager Tips Manager Specials News . Next Chelsea Manager Betting: Jose Mourinho backed in to 4/1 from 50/1.
no. of electrons in carbon|How to find the Number of Protons, Electrons, Neutrons for